Group Kemmerling
| ||||
Molecular analysis of Arabidopsis thaliana receptor protein kinases implicated in pathogen defense | ||||
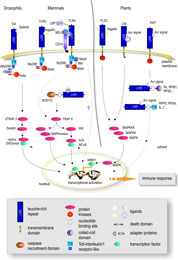
project LRR-RLKs are believed to form signal perception complexes with so far unknown interacting proteins. Since genes encoding components of signalling pathways in eukaryotes are often found to be transcriptionally up-regulated by the corresponding stimulus, we have exploited this to identify new LRR-RLK genes potentially involved in the activation of non-cultivar-specific as well as cultivar-specific plant defense. In a concerted approach to build up an expression atlas of Arabidopsis the ATGenExpress project gave us the possibility to perform array analyses on the expression patterns after pathogen and PAMP treatment. From a total of 235 LRR-RLK genes, 49 were found to be transcriptionally up-regulated upon either pathogen infection and/or elicitation (Affymetrix microarray analysis/RT-PCR verification). The molecular analysis of these genes will involve gene inactivation by T-DNA insertion/RNA interference, analysis of KO mutants in infection assays, studies on spatial and temporal gene expression and protein localization, and enzymatic activity of the kinase domains. Based upon phenotypic alterations (growth, development, responses to biotic and/or abiotic stress) in mutant plants (including changes in the transcriptome/proteome) selected LRR-RLKs will be subjected to identification of interacting proteins (co-immunoprecipitation/split-ubiquitin screen/Y2H). The analysis of interacting proteins as parts of functional networks will involve reverse genetics and biochemical characterization as above. | ||||
| ||||
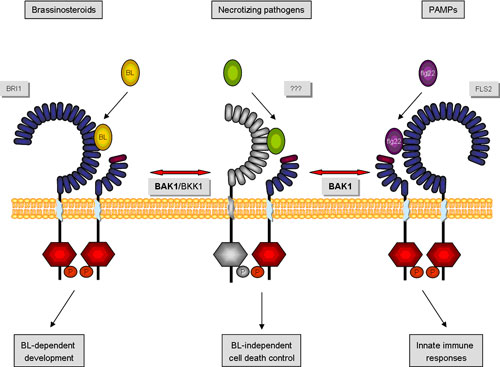
BAK1 and other DRKs (Defense-Related Kinases) | ||||
| ||||
| ||||
| ||||
publications
Imkampe J, Halter T, Huang S, Schulze S, Mazzotta S, Schmidt N, Manstretta R, Postel S, Wierzba M, Yang Y, van Dongen WMAM, Stahl M, Zipfel C, Goshe M B, Clouse S, de Vries S C, Tax F, Wang X, Kemmerling B. (2017) The Arabidopsis Leucine-Rich Repeat Receptor Kinase BIR3 Negatively Regulates BAK1 Receptor Complex Formation and Stabilizes BAK1. Plant Cell, 29(9):2285-303.
Rodiuc N, Barlet X, Hok S, Perfus-Barbeoch L, Allasia V, Engler G, Séassau A, Marteu N, de Almeida-Engler J, Panabières F, Abad P, Kemmerling B, Marco Y, Favery B, Keller H. (2015) Evolutionarily distant pathogens require the Arabidopsis phytosulfokine signalling pathway to establish disease. Plant Cell Environ., 39(7):1396-407.
Petutschnig EK, Stolze M, Lipka U, Kopischke M, Horlacher J, Valerius O, Rozhon W, Gust AA, Kemmerling B, Poppenberger B, Braus GH, Nürnberger T, Lipka V. (2014) A novel Arabidopsis CHITIN ELICITOR RECEPTOR KINASE 1 (CERK1) mutant with enhanced pathogen-induced cell death and altered receptor processing. New Phytol., doi: 10.1111/nph. 12920.
Halter T, Imkampe J, Blaum BS, Stehle T, Kemmerling B. (2014) BIR2 affects complex formation of BAK1 with ligand binding receptors in plant defense. Plant Signal Behav., 29;9. pii: e28944.
Blaum BS, Mazzotta S, Nöldeke ER, Halter T, Madlung J, Kemmerling B, Stehle T. (2014) Structure of the pseudokinase domain of BIR2, a regulator of BAK1-mediated immune signaling in Arabidopsis. J Struct Biol., 186(1):112-21.
Halter T, Imkampe J, Mazzotta S, Wierzba M, Postel S, Bücherl C, Kiefer C, Stahl M, Chinchilla D, Wang X, Nürnberger T, Zipfel C, Clouse S, Borst JW, Boeren S, de Vries SC, Tax F, Kemmerling B. (2014) The leucine-rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Curr Biol., 24(2):134-43.
Yang H, Postel S, Kemmerling B, Ludewig U. (2014) Altered growth and improved resistance of Arabidopsis against Pseudomonas syringae by overexpression of the basic amino acid transporter AtCAT1.Plant Cell Environ., 37(6):1404-14.
Mosher S and Kemmerling B#. (2013) PSKR1 and PSY1R-mediated regulation of plant defense responses. Plant Signal & Behav, 8(5):e24119. doi: 10.4161/psb.24119.
Tintor N, Ross A, Kanehara K, Yamada K, Fan L, Kemmerling B, Nuernberger T, Tsuda K, Saijo Y. (2013) Layered pattern receptor signaling via ethylene and endogenous elicitor peptides during Arabidopsis immunity to bacterial infection. Proc Natl Acad Sci, USA,110(15):6211-6.
Mosher S, Seybold H, Rodriguez P, Stahl M, Davies KA, Dayaratne S, Morillo S, Wierzba M, Keller H, Tax FE, Kemmerling B#. (2012) The Tyrosine-sulfated peptide receptors PSKR1 and PSY1R modify Arabidopsis immunity to biotrophic and necrotrophic pathogens in an antagonistic manner. Plant J, doi: 10.1111/tpj.12050.
Receptor-like Kinases in Plants: From Development to Defense (Signaling and Communication in Plants)Tax F and Kemmerling B. (Eds) Springer Verlag Heidelberg.
Kemmerling B#, Halter T, Mazzotta S, Mosher S, Nürnberger T# (2011) A genome-wide survey for Arabidopsis leucine-rich repeat receptor kinases implicated in plant immunity. Front. Plant Sci 2:88. doi: 10.3389/fpls.2011.00088.
Mazzotta S and Kemmerling B#. Pattern recognition in plant innate immunity, Journal of Plant Pathology, 2011, 93:7-17.
Humphry M, Bednarek P, Kemmerling B, Koh S, Stein M, Goebel U, Piślewska-Bednarek M, Loraine A, Schulze-Lefert P, Somerville S and Panstruga R. A complex regulon with a dual role in pathogen defense and development is conserved in monocot and dicot plants. PNAS 2010, 107(50): 21896-21901.
Krol E, Mentzel T, Chinchilla D, Boller T, Felix G, Postel S, Kemmerling B, Arents M, Jeworutzki E, Al-Rasheid K, Becker D, Hedrich R. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem 2010, doi/10.1074/jbc.M109.097394 in press.
Postel S, Küfner I, Beuter C, Mazzotta S, Schwedt A, Borlotti A, Halter T, Kemmerling B#, Nürnberger T#. The multifunctional leucine-rich repeat receptor kinase BAK1 is implicated in Arabidopsis development and immunity. Eur J Cell Biol 2010, 89:169-174.
Chinchilla D, Shan L, He P, de Vries SC, Kemmerling B#. One for all: the receptor-associated kinase BAK1. Trends Plant Sci 2009, 14(10):535-541.
Postel S and Kemmerling B. Plant systems for recognition of pathogen-associated molecular patterns. Semin Cell Dev Biol, 2009 DOI: 10.1016/j.semcdb.2009.06.002.
Krupnova T, Sasabe M, Ghebreghiorghis L, Gruber CW, Hamada T, Dehmel V, Strompen G, Stierhof YD, Lukowit W, Kemmerling B, Machida Y, Hashimoto T, Mayer U, Jürgens G. Microtubule-associated kinase-like protein RUNKEL for cell plate expansion in Arabidopsis cytokinesis. Current Biology 2009, 19(6):518-523.
Kumar M, Busch W, Birke H, Kemmerling B, Nürnberger T, Schöffl F. Heat shock factors HsfB1 and HsfB2b are involved in the regulation of Pdf1.2expression and pathogen resistance in Arabidopsis. Molecular Plant 2009, 2: 152-165.
Engelhardt S, Lee J, Gaebler Y, Kemmerling B, Haapalainen M, Li CM, Wei Z, Keller H, Joosten M, Taira S, Nürnberger T. Separable roles of the Pseudomonas syringae pv. phaseolicola accessory protein HrpZ1 in ion-conducting pore formation and activation of plant immunity. Plant Journal 2009, 57: 706-717.
Nürnberger T, Kemmerling B. Pathogen-associated molecular patterns (PAMP) and PAMP-triggered immunity. Annual Plant Reviews: Molecular Aspects of Plant Disease Resistance 2008, 34: 16-47.
Albrecht, C., Russinova, E., Kemmerling, B., Kwaaitaal, M. and de Vries, S. C. Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid-dependent and -independent signaling pathways. Plant Physiol 2008, 148(1): 611-619.
Kemmerling B, Nürnberger T. Brassinosteroid-independent functions of the BRI1-associated kinase BAK1/SERK3. Plant Signaling&Behavior 2008, 3(2):116 - 118.
Gust AA, Biswas R, Lenz HD, Rauhut T, Ranf S, Kemmerling B, Götz F, Glawischnig E, Lee J, Felix G, Nürnberger T. Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. J Biol Chem 2007.
Chinchilla D., Zipfel, C., Robatzek S., Kemmerling B., Nürnberger T., Jones, J.D., Boller T., A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defense. Nature 2007, 448(7152): 497-500.
Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Abu Qamar S, Mengiste T, Betsuyaku S, Parker JE, Müssig C, Thomma BPHJ, Albrecht C, de Vries SC, Hirt H, Nürnberger T. A Brassinolide-Independent Role for the BRI1-Associated Receptor Kinase 1 (BAK1) in Plant Cell Death Control. Current Biology 2007, 17: 1116-1122.
Qutob* D, Kemmerling* B, Brunner* F, Küfner I, Engelhardt S, Gust AA, Luberacki B, Seitz HU, Stahl D, Rauhut T, Glawischnig E, Schween G, Lacombe B, Watanabe N, Lam E, Schlichting R, Scheel D, Nau K, Dodt G, Hubert D, Gijzen M, and Nürnberger T. Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 2006,18(12): 3721-44.
*joint first-authorship
Nürnberger T., Kemmerling B., Receptor protein kinases – pattern recognition receptors in plant immunity. Trends Plant Sci 2006, 11: 519-22.
Consonni C, Humphry ME, Hartmann HA, Livaja M, Durner J, Westphal L, Vogel J, Lipka V, Kemmerling B, Schulze-Lefert P, Somerville SC, Panstruga R. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat Genet 2006, 38(6):716-20.
He P, Shan L, Lin NC, Martin GB, Kemmerling B, Nürnberger T, Sheen J. Specific Bacterial Suppressors of MAMP Signaling Upstream of MAPKKK in Arabidopsis Innate Immunity. Cell 2006; 125(3): 563-75.
Nürnberger T, Kemmerling B. Signal Perception and Transduction in Plant innate immunity, in: Communications in Plants. F. Baluska, S. Mancuso, D. Volkmann (Eds.) Springer Verlag Berlin Heidelberg 2006, 95-109.
Nürnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 2004; 198: 249-66.
Fellbrich G, Romanski A, Varet A, Blume B, Brunner F, Engelhardt S, Felix G, Kemmerling B, Krzymowska M, Nürnberger T. NPP1, a Phytophthora-associated trigger of plant defense in parsley and Arabidopsis. Plant J 2002; 32(3): 375-90.
Münch-Garthoff S, Neuhaus JM, Boller T, Kemmerling B, Kogel KH. Expression of beta-1,3-glucanase and chitinase in healthy, stem-rust-affected and elicitor-treated near-isogenic wheat lines showing Sr5-or Sr24-specified race-specific rust resistance. Planta 1997; 201(2): 235-44.
teaching
-
Comparative immunity in animals and plants
lecture
2 SWS
Di 17-19 Uhr wöchentlich
ZMBP Morgenstelle 32, Seminarraum 4U09 -
Comparative immunity in animals and plants
seminar
2 SWS
Zeit nach Vereinbarung
ZMBP Morgenstelle 32, Seminarraum 4U09 -
Literaturseminar und Seminar über aktuelle Forschungsarbeiten des Instituts
2 SWS
Di 9-11 Uhr, wöchentlich
ZMBP, Morgenstelle 32, Seminarraum 4U09 -
Praktikum Molekulare Mechanismen der angeborenen Immunität
10 SWS, ganztägig;
Voranmeldung erforderlich bei Dr. B. Kemmerling
(Tel. 29-76654) -
Projektmodul (Arbeitsgruppenpraktikum) für Biochemiker
ganztägig 5-6 Wochen;
Voranmeldung erforderlich bei Dr. A. Gust, Dr. B. Kemmerling
(Tel. 29-76654/55) - Bereichsmodul für Bachelor Biologie/Biochemie
ganztägig, 4 Wochen
ZMBP Morgenstelle 32, 5N22
Voranmeldung bei Dr. B. Kemmerling (Tel. 29-76654)
- Zellbiochemie und SIgnaling Biologie/Biochemie
2-wöchig ganztägig,
ZMBP Morgenstelle 32, Praktikumsraum 3U11
Beginn: Mo 26.01.15 10:00 s.t.
-
Biochemisches Praktikum für Biologen
4 SWS, ganztägig 10-16 Uhr
Medizinerpraktikumssaal IFIB, Hoppe-Seyler-Str. 4
Beginn: Di 29.07.14,, 10 s.t. Gr. Hörsaal Vorbesprechung
co-operations
Delphine Chinchilla, Thomas Boller, Basel
Sacco de Vries, Wageningen
Cyril Zipfel, Norwich
Frans Tax, Tucson
Steven Clouse, Raleigh
Jeff Dangl, Chapel Hill
Heribert Hirt, Evry
Klaus Harter, Tübingen
Erich Glawischnig, München
Claus Wasternack, Halle
Bart Thomma, Wageningen
Friedrich Schöffl, Tübingen
Tesfaye Mengiste, West Lafayette
Jane Parker, Köln
Mahmut Tör, Warwick
BASF
people
We thank the DFG and EU for funding of our projects:
AFGN;
Bravissimo, FP7;
ERA-PG;
SFB446
BMBF-NRF
DFG KE1485/1-1
DFG HA2146/1-1 with group Harter
SFB1101 D3