Prof. Dr. Claudia OeckingUniversität Tübingen
ZMBP, Pflanzenphysiologie
Auf der Morgenstelle 32
D-72076 Tübingen / Germany
claudia.oeckingspam prevention@zmbp.uni-tuebingen.de
++49-7071/29-76679Secretaries
Elke Fischer
Tue-Fri 1pm - 6pm
++49-7071/29-78802
elke.fischerspam prevention@zmbp.uni-tuebingen.de
++49-7071/29-3287
Nicole Doppstadt
Tue, Thu & Fri 2pm - 6 pm
++49-7071/29-73225
ppspam prevention@zmbp.uni-tuebingen.de
++49-7071/29-3287
Not occupied on Mondays.
Function of plant 14-3-3 proteins
Reversible phosphorylation of proteins is the most widespread signaling mechanism and regulates almost all aspects of cellular life. 14-3-3 proteins were the first molecules to be identified as discrete phosphoserine/ threonine binding modules and are highly conserved in both the animal and plant kingdoms. Coordination of a client protein within the amphipathic groove of a 14-3-3 dimer can have a range of contextdependent effects such as conformational change, re-localization and bridging of two molecules. Accordingly, 14-3-3 homologs seem to complete signalinduced and phosphorylation-dependent transitions in protein activity and have emerged as key modulators of signal transduction events.
Research in my laboratory aims to understand the biological functions of 14-3-3 proteins in higher plants. In addition to protein crystallization and structure determination, an array of biochemical, molecular and cell biological approaches is applied.
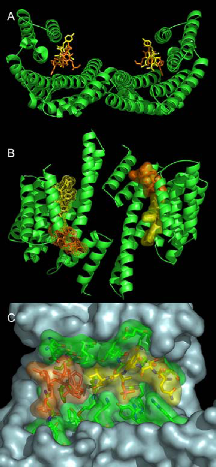
We have identified 14-3-3 proteins as positive regulators of the plant plasma membrane H+-ATPase that provides the driving force for nutrient uptake and maintenance of cell turgor. The enzyme is kept at a low activity level by its C-terminal domain, the autoinhibitory action of which is released upon binding of 14-3-3 proteins. According to our data the enzyme’s C-terminal domain harbors two nonclassical sites for regulatory 14-3-3 proteins: the phosphorylated extreme C-terminal end (YpTV-COOH) and an unphosphorylated motif located further upstream. The established complex represents the target for the wilt-inducing phytotoxin fusicoccin (FC), a well known activator of the pump in vivo. The molecular action of this diterpenglucosid was uncovered by X-ray analysis (Fig. 1). In brief, FC closes a gap that remains in the binding groove after coordination of the atypical phosphorylated binding motif of the H+-ATPase leading to mutual stabilization as well as increase in binding affinity of both ligands. As a consequence of permanent activation of the H+-ATPase, stomatal pores are irreversibly opened followed by wilting of plants.
Fig. 1: The ternary 14-3-3/phosphopeptide/FC complex. Ribbon plot (A, B) of the phytotoxin FC (orange) and a phosphorylated pentapeptide representing the plant H+-ATPase’s extreme C-terminal end (QSYpTV-COOH; yellow) coordinated within a 14-3-3 dimer (green). (B) shows the view rotated by 90° about the horizontal axis. (C) Van der Waals surface representation of one amphipathic 14-3-3 binding cleft showing the close contact of both ligands.Current results show that a 14-3-3 dimer is simultaneously occupied by two entire binding motifs of the H+-pump – each consisting of two nonclassical sites - in an antiparallel fashion and an unusual conformation. Furthermore, the structure suggests an – up to now – unidentified mode of 14-3-3 action: association seems to induce the assembly of an active H+- ATPase oligomer. Future experiments aim at elucidating the relevance of 14-3-3 proteins with respect to oligomerization. In addition, we have established an appropriate method for the identification of the protein kinase(s) mediating this important regulatory interaction.
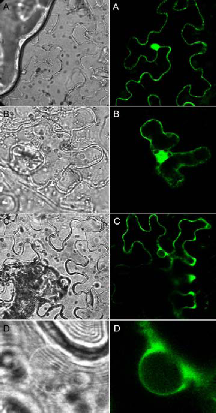
In order to address the question of 14-3-3 function at the level of the whole organism, we have started to analyze gene expression patterns of individual 14- 3-3 isoforms as well as their subcellular localization. For this purpose genomic GFP fusions of all thirteen Arabidopsis isoforms will be studied for expression in transgenic plants. In addition we have established a proteomic approach to identify novel target polypeptides whose interaction with 14-3-3 proteins will be studied at the molecular and cellular level. In this regard, interaction with the glycolytic enzyme enolase as one identified target was verified both in vitro (immunoprecipitation, affinity chromatography) and in vivo (bimolecular fluorescence complementation, BiFC). BiFC represents a novel method for direct visualization of protein interactions in living cells and is based on complementation between two nonfluorescent fragments of the yellow fluorescent protein (YFP). Interestingly, functional reconstitution of YFP was exclusively observed in the cytoplasm (Fig. 2 C, D) while homodimerization of the corresponding polypeptides (14-3-3: Fig. 2A, enolase: Fig. 2 B) could be additionally detected in the nucleus. Glycolytic enzymes are often considered rather dull enzymes. However, recent findings have shown that the enolase is bi-functional in that it additionally functions as a transcription factor required for expression of cold-responsive genes. Taken together, 14-3-3 binding possibly sequesters enolase in the cytoplasm and the release of 14-3-3 then allows the enzyme to relocate to the nucleus.
Last but not least, reverse genetics as well as manipulation of the expression of 14-3-3 homologs aims at elucidating their physiological roles and isoform-specific functions.
Fig. 2: BiFC visualization of protein-protein interactions in Agrobacterium-infiltrated Nicotiana benthamiana leaves. Confocal (right panel) and bright field (left panel) images of epidermal leaf cells are shown. (A) Homodimerization of 14-3-3, (B) homodimerization of enolase, (C) interaction between enolase and 14-3-3, (D) enlarged view of the nucleus shown in (C) demonstrating that association is restricted to the cytoplasm.